When “calorie” is mentioned, thoughts typically drift toward diet plans, food labels, and weight control. However, the significance and application of calories stretch far beyond the realm of nutrition, rooting deeply in the principles of chemistry.
This post will comprehensively explore the science of calorie in chemistry, the history behind its conception, the fundamental science involved, and its pertinence to everyday life.
1. Introduction to Calorie: Chemistry Meets Nutrition
A calorie is a unit of measurement used to determine the amount of energy the human body can use in food and drinks. In scientific terms, specifically in chemistry, one calorie is defined as the energy required to raise the temperature of one gram of water by one degree Celsius.
This fundamental concept is pivotal in understanding how energy is stored and utilized in various substances, including foods. From a nutritional standpoint, calories are essential for managing body weight and ensuring that individuals consume the right energy to fuel their daily activities.
The balance between calories consumed and calories expended through physical activities ultimately dictates weight gain, loss, or maintenance.
“In exploring the calorie, we bridge the gap between the abstract world of chemical energy and the tangible aspects of food consumption. It’s a fascinating intersection where science meets daily life,” explains Dr. Alex Thompson, a nutrition scientist. “Acknowledging the dual role of calories enriches our scientific comprehension and empowers us to make informed dietary choices.”
Read More: How Many Calories in 1g of Carbohydrate? A Comprehensive Guide
2. Small Calorie vs. Large Calorie
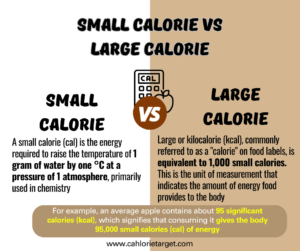
The distinction between a small calorie (cal) and a large calorie (Cal), also known as the kilocalorie (kcal), is crucial for understanding nutritional information. A small calorie (cal) is the amount of energy required to raise the temperature of 1 gram of water by one °C at a pressure of 1 atmosphere, primarily used in chemistry.
In contrast, the large or kilocalorie (kcal), commonly referred to as a “calorie” on nutrition facts labels or food labels, is equivalent to 1,000 small calories. This is the unit of measurement that indicates the amount of energy food provides to the body.
For example, an average apple contains about 95 significant calories (kcal), which signifies that consuming it gives the body 95,000 small calories (cal) of energy. This distinction is vital for accurately measuring and understanding the energy content in foods to keep a healthy diet.
3. What is the Energy Content in Calories?
In essence, the calorie serves as a fundamental unit of energy, pivotal both in the laboratory for chemical experiments and daily life for gauging food energy. This dual role underscores the versatility of the calorie, acting as a crucial link between the abstract concepts of energy in the realm of chemistry and the tangible aspects of nutrition that affect our health and wellbeing.
For example, when we consider the energy content of an apple, which is approximately 95 kcal (or 95,000 calories in the chemical sense), we’re not just talking about its potential to fuel our body’s metabolic processes.
Instead, we’re also referencing the chemical energy stored within the apple’s molecules—that, through digestion and metabolism—can be converted into kinetic energy for physical activities or thermal energy to maintain body temperature. To maintain a healthy weight, it’s important to monitor your calories per day and ensure they align with your body’s needs and activity level.
Read More: How to Make Low Calorie Bread?
4. Calories in Everyday Life: From Food Choices to Exercise
Understanding calories from a chemistry standpoint elevates our comprehension of food energy. It explains why certain foods are more ‘energy-dense’ than others and how our bodies utilize these calories for vital functions and physical activities. This knowledge empowers individuals to make informed food choices and adopt exercise routines that align with their energy needs.
5. Looking Ahead: The Future of Calorie Measurement
The method of measuring calories in food, currently based on bomb calorimetry tests that determine the energy content by burning the food, is evolving. Innovations in nutritional science and technology promise more accurate measurements and personalized approaches to dietary energy estimation. This evolution underscores the importance of continuous learning and adaptation in both chemistry and nutrition.
Dr. Michael Matthews, a renowned Nutrition Scientist, emphasizes, “The future of understanding calories in nutrition hinges on our ability to integrate chemical principles with biological complexities. This integration will advance our approach to diet and wellness.”
Read More: What is an Active Calorie: Your Guide to Enhanced Fitness and Weight Management
6. How many calories do I need to burn to lose weight (chemistry explanation)
The concept of burning calories to lose weight merges the realms of chemistry and personal health, offering a fascinating insight into how energy balance affects our bodies. In chemistry, losing weight through calorie burning is a process of energy expenditure exceeding energy intake.
When we engage in physical activities, our bodies convert stored chemical energy—primarily from fat and carbohydrates—into kinetic energy for movement and thermal energy to maintain body temperature. This conversion process requires the breakdown of molecules, releasing stored energy as heat and work done by muscles.
Prof. Alexander Fischer, a prominent chemist, adds, “The calorie is more than a number on a nutrition label; it’s a fundamental concept in energy transfer that underscores the interconnectedness of chemical reactions and life processes.”
To lose weight, the body’s energy must surpass what is consumed as food and drink. This deficit forces the body to metabolize stored fat, resulting in weight loss. This understanding empowers us to make informed decisions regarding diet and exercise to achieve our weight management goals.
7. Are All Calories Created Equal? (Fitness and Chemistry)
Whether all calories are created equal is a significant debate among nutritionists and chemists. From a purely chemical standpoint, a calorie is a unit of energy. However, the waters muddy considerably when we enter nutrition and metabolic biochemistry.
Different nutrients (carbohydrates, fats, and proteins) undergo distinct metabolic pathways in the body, each with its efficiency and energy release rate. Furthermore, the thermic effect of food (TEF), which represents the energy expended during digestion, absorption, and metabolism of nutrients, varies among macronutrients.
Protein, for instance, has a higher TEF than carbohydrates and fats, meaning it requires more energy to metabolize. This discrepancy suggests that the source of calories matters as much as the calorie count in the context of weight management and overall health.
Foods with the same caloric value can have vastly different effects on hunger, hormonal responses, and metabolic outcomes. Therefore, while the chemical definition of a calorie remains constant, its biological implications are far more complex and nuanced.
Read More: Unlocking the Sizzle: How do you Make Steak Tender and Juicy
8. Key Takeaways
A balanced approach to calories, informed by chemical and nutritional aspects, is essential for health and well-being. While calories serve as a basic unit of energy in dietary contexts, their chemical roots offer a deeper understanding of energy efficiency in the physical world.
By appreciating the calorie’s dual nature, we can better navigate the intricate balance of energy input and output that governs metabolic pathways and technological advancements.
In conclusion, exploring calories in chemistry highlights the intricate dance between energy measurement and utilization, propelling us toward a future
